 Relevancy and Engagement
agclassroom.org/ne/
Relevancy and Engagement
agclassroom.org/ne/
Lesson Plan
Matter of Fact
Grade Level
Purpose
In this lesson, students will take on the role of a nitrogen molecule and experience how various forms of nitrogen cycle through the environment. Students will be able to identify and differentiate between atoms, molecules, and compounds. Grades 9-12
Estimated Time
Materials Needed
For the teacher:
- Document projector
- Transparency film (optional)
- Tactile molecular models
- Matter of Fact Notes handout and answer key
- What Goes Around, Comes Around answer key & station descriptions
- Molecular Shuffle slides
For each station:
- Station instructions
- Die
- Twenty toothpicks
- Bowl
- Twenty gumdrops (various colors)
For each student:
Vocabulary
ammonification: bacteria or fungi convert organic forms of nitrogen into ammonium, which can be used by plants
denitrification: under poor aeration, soil bacteria convert nitrate ions into nitrogen gas which cannot be used by plants and is lost to the atmosphere
nitrification: to break down nitrogen compounds to nitrites and nitrates by bacterial action
nitrogen: a colorless, odorless unreactive gas that forms about 78% of the earth's atmosphere
Background Agricultural Connections
This lesson is one in a series of related lessons to introduce students to chemistry and environmental science concepts. Activities are modeled after real-life challenges that modern farmers face while producing our food, fiber, and fuel. Labs are inquiry based and promote critical thinking skills. Other related lessons include:
All organisms require nitrogen to live and grow; it is a fundamental component of DNA and RNA, the building blocks of life. Approximately 78% of the Earth’s atmosphere is made of nitrogen gas. This atmospheric form (N) is unusable by most plants. In order to be used by plants, nitrogen gas must be converted to ammonium, nitrate, or urea through a process called nitrogen fixation.
The nitrogen cycle is the process by which nitrogen is converted between its various chemical forms as it moves between the atmosphere, living organisms, and the Earth’s crust. The cycle illustrates how nitrogen from the atmosphere interacts with microorganisms that can convert, or “fix,” N gas into forms of nitrogen that are usable by plants. Nitrogen can also enter the cycle from other sources besides the atmosphere including manure, decaying plant material, and commercial fertilizers. As nitrogen atoms move throughout the cycle, their chemical composition may change numerous times.
Forms of Nitrogen Highlighted in the Lesson:
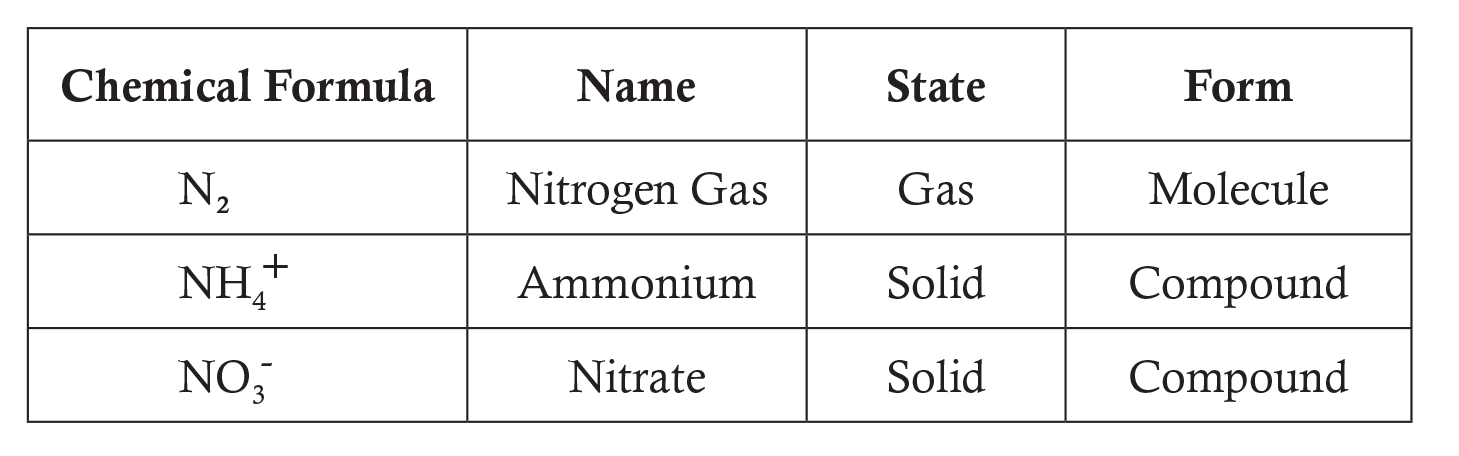
For more information see Answers to Commonly Asked Questions.
Engage
- Explain to students that many of the world’s resources are not available for use by plants or animals. For example, only one percent of the Earth’s water is actually drinkable. Have students brainstorm reasons why the other 99 percent might not be available for consumption. Record answers on the board. After some time, explain that 97 percent of water on Earth is salt water. Two percent of the water on earth is glacier ice at the North and South Poles.
- Similar to the availability of water, some plant nutrients are not easily used by plants. For example, although 80 percent of the Earth’s atmosphere is made of nitrogen—this form (N or nitrogen gas) is unusable by plants. Today we’re going to experience how nitrogen changes its molecular form during the nitrogen cycle and learn what forms can be assimilated by plants.
- In this lesson, students will:
- learn about the nitrogen cycle as they take on the role of a nitrogen molecule as it cycles through the environment; and
- differentiate between atoms, molecules, and compounds.
Explore and Explain
Part I:
- Prior to the lesson, prepare several tactile molecular models to represent each of the following forms of nitrogen: N, N, NH4+, NO3-. Display for the class the Matter of Fact Notes handout and the Nitrogen Cycle diagram onto overhead transparencies (optional). Set up seven stations evenly spaced around the classroom. Place a bowl of gumdrops, toothpicks, and a die at each station. Post the station instructions above each station.
- Distribute the Matter of Fact Notes handout. Complete the handout with the class. As you review the different forms of nitrogen, show students examples using molecular models. Identify the type of atoms in each molecule.
- Show students the Nitrogen Cycle diagram. Review each step of the nitrogen cycle.
- Introduce the activity, What Goes Around, Comes Around. Explain to students that there are seven different stations around the classroom. Each station represents a “reservoir” for nitrogen in the nitrogen cycle. Tell students that in this activity they will act as nitrogen atoms moving through the nitrogen cycle. They will start as a pure form of nitrogen. In this form, they are a single atom. Hold up a single gumdrop. At each station students will role a die to determine how they will transform. Students will create a model of the different forms of nitrogen using the toothpicks and gumdrops provided at their starting station. They will take this model with them as they transform into different forms of nitrogen at each station. Have extra gumdrops on hand for construction of the models. Each color gumdrop will represent a different element, the toothpicks will represent chemical bonds. Students should record the information from each station on their What Goes Around, Comes Around chart. Students may use the Nitrogen Cycle handout to identify each process in the cycle. Briefly review each station and tell students that you will give the signal to switch stations every five minutes and that they should rotate to different stations as determined by the roll of the die at each of their stations.
- Instruct students to return to their desks and complete the "Think About It" section of the handout. Students may work in pairs. Answer any questions before releasing students to begin the activity.
- As a class, review the "Think About It" section of the handout. Lead a class discussion to highlight the following “big picture” concepts:
- There is a limited amount of nitrogen in the environment. Nitrogen changes forms as it moves through different stages of the nitrogen cycle.
- Nitrogen does not move through the cycle as a single atom, but in stable compound and molecule forms.
- Bacteria play an important role in ammonification, denitrification, fixation, and nitrification. Without bacteria, the nitrogen cycle would cease to be a productive cycle.
Part II: Review
- Prior to the review activity, divide the class into two equally sized groups based on an observable trait. For example, distribute paper streamers or divide the group by gender.
- Introduce students to the review activity, Molecular Shuffle. Explain that in this activity, you will reveal different molecular formulas, including atoms, molecules, and compounds. After each chemical formula is revealed, students will scramble to create a group that accurately represents the atoms in the chemical formula.
- For example, if the formula NO (nitric oxide) is revealed, a student with a green paper streamer will link arms with a student who has a blue paper streamer, to represent the two different elements bonded together. If the formula is HO (water) two green and one blue or two blue and one green will link together. If a student is unable to find a group they are “out” and can watch the activity from the sidelines. Any incomplete groups are also “out.” If you announce a single atom, the students must stand at attention and yell “I’m an atom!”
- Play the review activity, incorporating each form of nitrogen. Supporting presentation slides for the Molecular Shuffle can be found in the Essential Files portion of this lesson.
Variations
- Instruct students to use arrows and symbols to record their movement through the nitrogen cycle. Combine each student’s unique path using different colors on a shared class diagram.
- Make the review activity, Molecular Shuffle, increasingly challenging. As students become familiar with the different forms of nitrogen, call out the name of the molecule only. Students must determine the chemical formula quickly before gathering into groups. Add oral responses for students to categorize elements as micro or macro nutrient, or increase the complexity of chemical formulas, using examples like calcium nitrate Ca(NO).
ELL Adaptations
- The addition of the complex terms and science concepts can make learning even more difficult. Write down key terms so students can see them and connect them to the spoken word.
- Demonstrate activities in front of class to ensure that English language learners can see the procedures before engaging in an activity. Pair ELL students with partners who are English proficient and have a good understanding of topics being taught in class.
Elaborate
-
Have students plan and construct a three-dimensional nitrogen cycle diagram using common household items or craft supplies
-
Watch an educational video that illustrates the nitrogen cycle. Search YouTube using the term “Nitrogen Cycle.”
-
Have students create a “mind map” to visually organize information. Several online tools, such as www.mindmeister.com and www.mindomo.com offer real-time collaboration in order to have a concept map that the whole class can edit at the same time.
Evaluate
After conducting these activities, review and summarize the following key concepts:
- Though the earth has many resources, the available resources to produce our food such as fertile soil and water are limited.
- Some nutrients such as the gaseous form of Nitrogen are abundant, but not useable for plant growth.
- Natural cycles, such as the Nitrogen Cycle govern the flow of nutrients.
- Farmers use their knowledge of science and biology to produce our food supply.
Acknowledgements
This lesson was funded in 2011 by the California Department of Food and Agriculture’s (CDFA) Fertilizer Research and Education Program (FREP). Chemistry, Fertilizer, and the Environment was designed to reinforce chemistry and environmental science concepts while educating students about the relationships between food, plant nutrients, farmers and the environment.
Executive Director: Judy Culbertson
Illustrator: Erik Davison
Layout and Design: Nina Danner